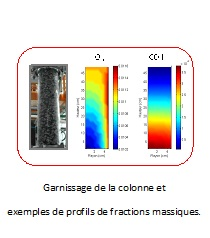
Reactive absorption
Work on reactive absorption began in September 2009. The porous medium is the inert column packing with the coexisting multicomponent gas and liquid phases that are involved in instantaneous homogeneous reactions. Unlike kinetic reaction terms, phase change volume rates and instantaneous reaction terms are not expressed as a function of independent variables. Therefore, these are handled by supplementing equilibrium equations which complement the conservation equations. The results obtained for the reactive absorption of carbon dioxide in an aqueous sodium hydroxide solution on a bulk column packing are in agreement with conventional observations. However, a realistic simulation of the process requires many modifications.
Here, as in the case of waste gasification, the boundary conditions of the packing must be computed by modeling and simulating the environment.
The thermodynamic aspect was simplified using three hypotheses: the liquid phase is an ideal solution, the gas phase is an ideal gas mixture and the liquid phase density is constant. The laboratory has the required models (compressibility factor, activity coefficients, fugacity coefficients, excess enthalpy values, etc.) for the fluids in the application, so the fluid phases can be processed as real phases.